Share
Australian ophthalmologists and researchers have made significant inroads into managing glaucoma. Professor William Morgan from Lions Eye Institute, University of Western Australia, was integral to the development of a microfistula tube, now known as the ‘XEN gelatin’ implant.
In 1995, Professor Dao-Yi Yu, a renowned ophthalmologist and researcher, proposed to develop a new form of glaucoma surgery. He led an innovative team at the Physiology and Pharmacology Centre and the McCusker Glaucoma Centre, Lions Eye Institute, the University of Western Australia. At the time, I was a PhD student studying pressure gradients in the optic nerve and Prof. Yu was my main supervisor. He prepared a patent (Yu and Morgan: Biological Microfistula Tube and Implantation Method and Apparatus AU#721915 (1996), PCT/AU97/00811 (1997), US patent #6544249) and invited me to be a co-inventor and join the project.
This project was to develop a minimally invasive surgical technique and a drainage implant that matched physiological conditions as much as possible. To achieve this goal, he developed a bioengineered implant using cross-linked gelatine to make a tiny stent called a microfistula. Additionally, he developed an ab-interno procedure via the anterior chamber avoiding damage to conjunctiva, and built a special implanter. The microfistula was to be injected across the trabecular meshwork, straddling the space between the anterior chamber and the conjunctival regions. Ideally, this would create a drainage bleb without damaging the conjunctiva or requiring scleral incisions.
Developing The Microfistula And Implanter
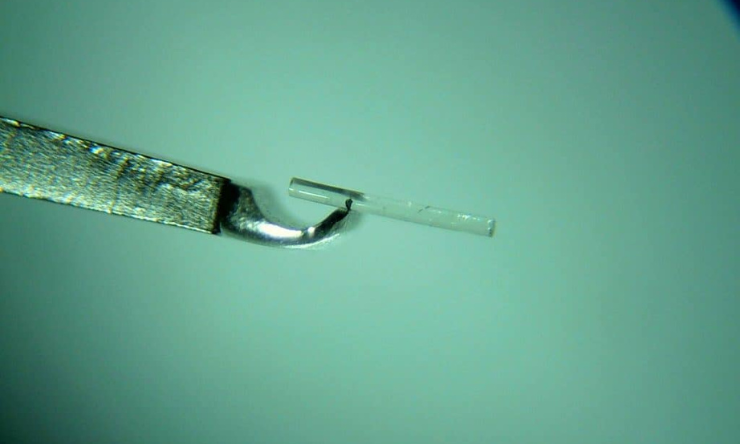
The first step was to develop the microfistula and implanter. Developing the microfistula tube was a real challenge because it needed to be bio-compatible and small enough in size to match physiological resistance. It also needed to be semi-rigid when implanting, then soften and swell after implantation. We developed custom- built bench equipment and made thousands of implants using various gelatine cross- linking parameters, and did more than 400 test implantations in animals. Finally, we were able to meet all requirements and worked out how to adjust the cross- linking parameters to control the time of microfistula absorption in the eye.
”We have to place this tiny tube in the right location, without damage to cornea or ciliary body and without touching the corneal endothelium or the iris in the angle”
At same time, Prof. Yu was working with a strong engineering team to build the implanter and manipulation system. We were fully aware that the microfistula had to be precisely placed in the right position, with consistent timing. To avoid possible variations made by the surgeon, we decided to use a motorised implantation and robotic manipulation and only one surgeon (Prof. Yu) did all the implantation surgery in animals. We effectively avoided surgical variations, which provided us an opportunity to identify the key parameters for improving the surgical outcomes. A motorised implanter with a controller and robotic system was specially built (Figure 1A and 1B). The motorised implanter allowed the implantation time to be adjusted to match the swelling of the microfistula tube during implantation.
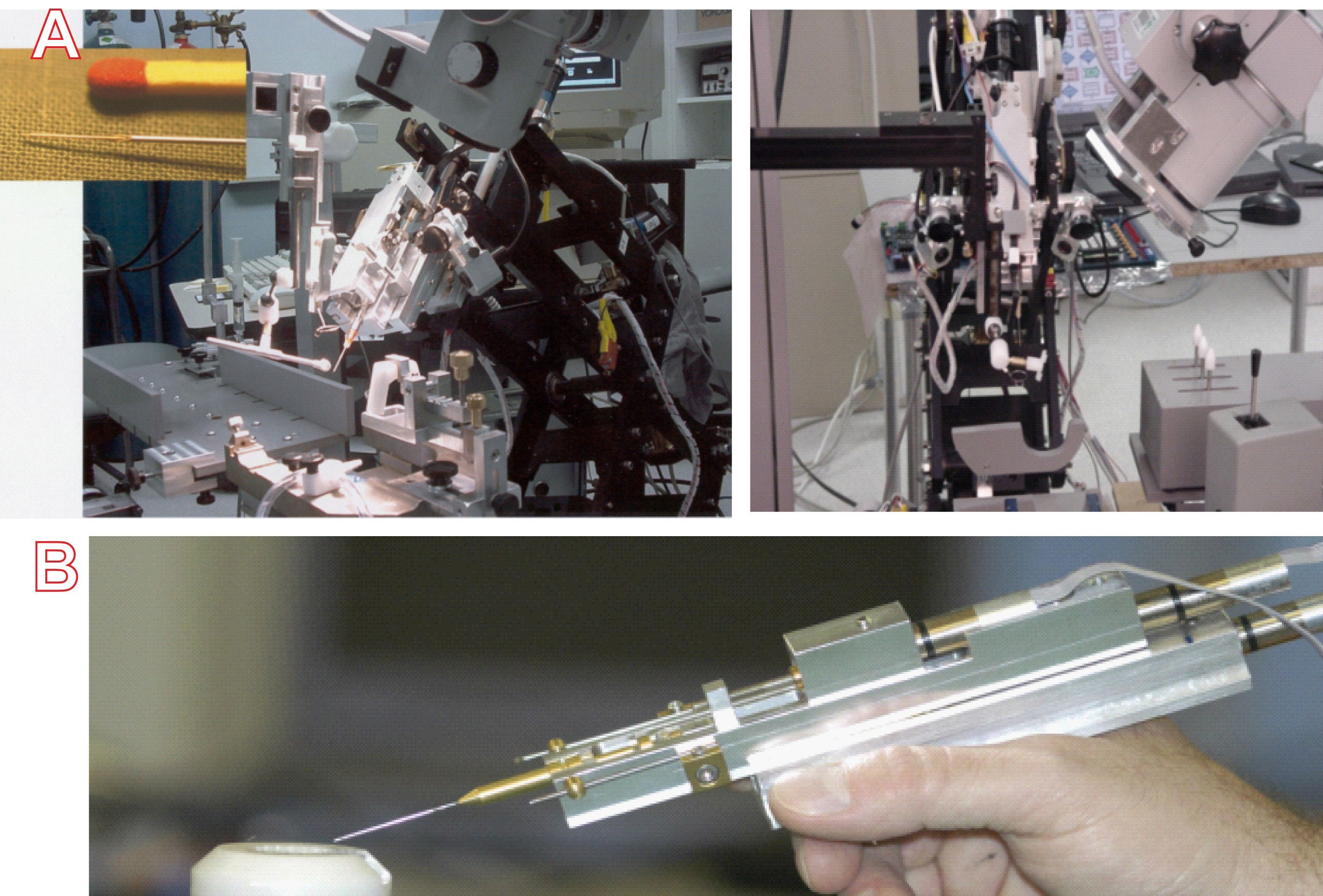
Figure 1. The robotic manipulation system and motor controllers. A. Insert of a magnified view of the microfistula ready for implantation. B. An early version of the motorised hand held implanter.
To speed up the project, we invited Prof. Xinghuai Sun’s team (Eye & ENT Hospital, Shanghai, China) to join with our Perth team. This allowed extensive animal experiments with follow-up times as long as six years, and provided much new and important knowledge. We obtained valuable information regarding the biological compatibility and longevity of the cross-linked material in the eye. We also demonstrated that our concept and technique could be a safe, effective and minimally invasive surgery for glaucoma.
Overcoming Challenges
An interesting finding from the experimental studies was that even after a trouble free implantation, some blebs failed within two weeks. This led us to think about why there could be different outcomes, even with a good implantation.
It became clear there were some other factors playing an important role… we closely monitored the drainage channel and bleb after surgery and found the failure occurred in the conjunctiva. This meant that filtration surgery could fail, even when a perfect drainage channel was created from the anterior chamber to the conjunctiva. We knew this question had to be addressed because it is critical, not only for our microfistula surgery, but for any glaucoma filtration surgeries including trabeculectomy and other implants. We found that almost all long-term functional blebs had active lymphatic drainage. We attempted to place the microfistula tube in locations rich in lymphatics and achieved functional blebs lasting more than six years in animals, that is the longest longevity reported. The longevity of the functional blebs in animals with active lymphatic drainage is at least six times longer than that without lymphatic drainage. Critically, we had discovered the important roles of the conjunctival lymphatics in the outcome of glaucoma filtration surgery. Our discoveries were published in Progress in Retinal and Eye Research, the highest ranking journal in ophthalmology. All the authors in this paper contributed their best efforts to make a success of this project. We also acknowledge the people from the engineering team who made remarkable contributions. This project could not have succeeded without them!
Moving To Commercialisation
After 10 years of hard and successful work, we wished to translate our experimental study to clinical application through commercialisation. In 2006, the Lions Eye Institute, who owned the patents, negotiated with The Innovation Factory, a US based incubator to setup a start-up company, AqueSys in California to purchase exclusive rights to the patents in agreement for certain milestones in terms of the development of the system. Prof. Yu’s team has transferred all their techniques, information and knowledge to AqueSys, and helped them to get substantial funding from venture capitalists. His team was also a part of the subsequent international clinical trial across seven countries.
A significant achievement by AqueSys was to convert the lab-based microfistula manufacturing procedure to an industrial scale, machine based procedure, that allowed the finished product to be standardised. Prof. Yu provided AqueSys with details of the step-by-step procedures and cross-linking protocols for making a long lasting microfistula in the living eye. His team tested the important parameters in lab bench and animal studies to ensure quality standards. AqueSys named the microfistula tube the ‘XEN gelatin’ implant. The company improved the hand held motorised implanter and ran international multiple centre clinical trials, then made continuous improvements to the implanter systems, finally arriving at a hand operated disposable product.
We wish to acknowledge all the ophthalmologists who contributed their clinical experience and knowledge, particularly in providing valuable suggestions throughout this project. Prof. Yu analysed all patients from the clinical trial and determined the optimum procedures to achieve the best outcomes. We learned that although this procedure appears ‘simple’, in reality it is far more complex. We have to place this tiny tube in the right location, without damage to cornea or ciliary body and without touching the corneal endothelium or the iris in the angle. The distal end of the microfistula needed to be placed in the superficial layer of Tenon’s capsule at a certain distance from the limbus.
First Human Procedure
We performed our first human microfistula procedure in 2007. I was particularly nervous about doing a novel procedure using novel material on a living patient of mine. Both Dao-Yi Yu and I wanted to watch this particular patient very closely for quite a few months before we embarked on more procedures in case there were problems. Fortunately, this particular patient, who had failed trabeculectomy before, did extremely well with a pre-operative pressure of 25 mmHg coming down to 8 mmHg and virtually remaining at 8 mmHg for the entirety of the follow-up period, which was three years. However unfortunately, that particular patient died of cardiovascular disease three years after surgery.
All of our early patients, bar one, had undergone previous glaucoma surgery, either trabeculectomy and/or Molteno tube surgery: and all bar two had undergone prior cataract extraction surgery. The rationale was that trabeculectomy was still the gold standard procedure and that patients who had not previously had trabeculectomy should be offered that primarily. Only when it had failed did we feel justified in offering this novel procedure.
We began to do more patients under a sponsored FDA Phase 1 and Phase 2 Study between 2009 and 2010, performing 23 procedures on 21 subjects (Figure 2). Following the success of the early studies, AqueSys enlarged the multicentre study to various centres in Europe and North America and concentrated on the major ophthalmic markets. The trial data was later accepted by the United States Food and Drug Administration. Full European CE certification was granted in 2014 and since then, more than ten thousand XEN implants have been performed in Europe. Full FDA approval was granted in the United States in 2016 followed by Canadian and New Zealand approval. In 2016, Allergan Inc. took over the manufacture, sales and distribution of the XEN gelatin microfistula. Recently, two-year data from a randomised multicentre study suggested that there is comparability in intraocular pressure outcomes between trabeculectomy and XEN microfistula insertion.
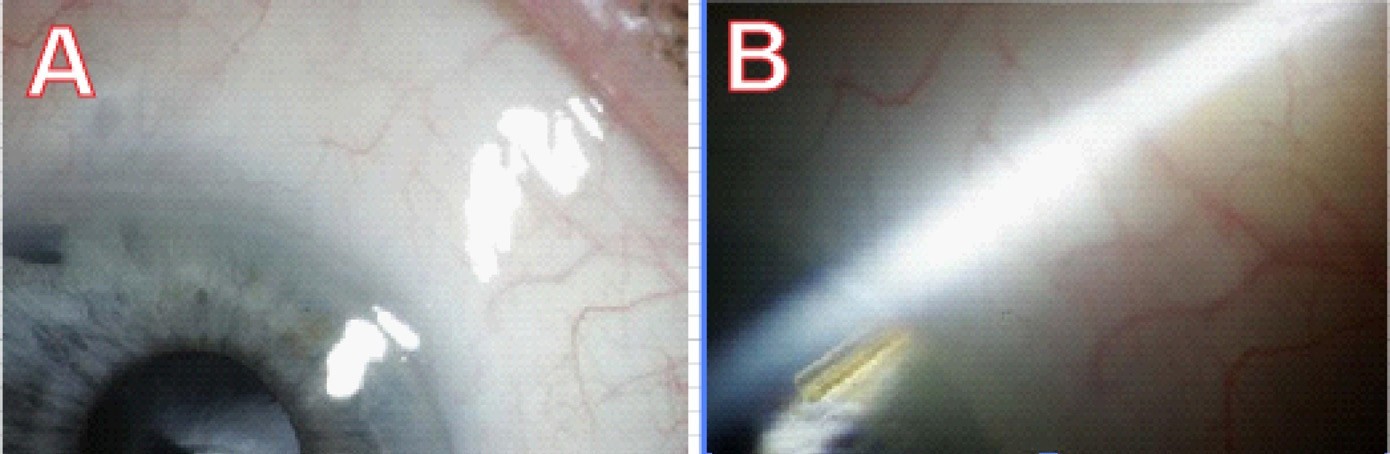
Figure 2. An implanted microfistula tube in the first human eye surgery, two years postoperatively. A. The conjunctiva shows a shallow bleb and looks healthy. B. The microfistula entering the angle of the eye.
Further Developments
To achieve the optimum outcome from each patient, we have to work to improve XEN- Microfistula surgery. Obviously, we urgently need to develop a non-invasive and label free imaging technique to help the surgeon find the optimum location for the XEN- Microfistula to be implanted, and then monitor the interaction between aqueous humour and lymphatic capillaries in the conjunctiva. Prof. Yu’s team is currently working with engineering teams to develop the required non-invasive conjunctival lymphatics imaging techniques.