Share
Melbourne-based ophthalmology company, PolyActiva, has reported promising clinical trial results for its six-month sustained drug delivery, biodegradable ocular implant in glaucoma patients.
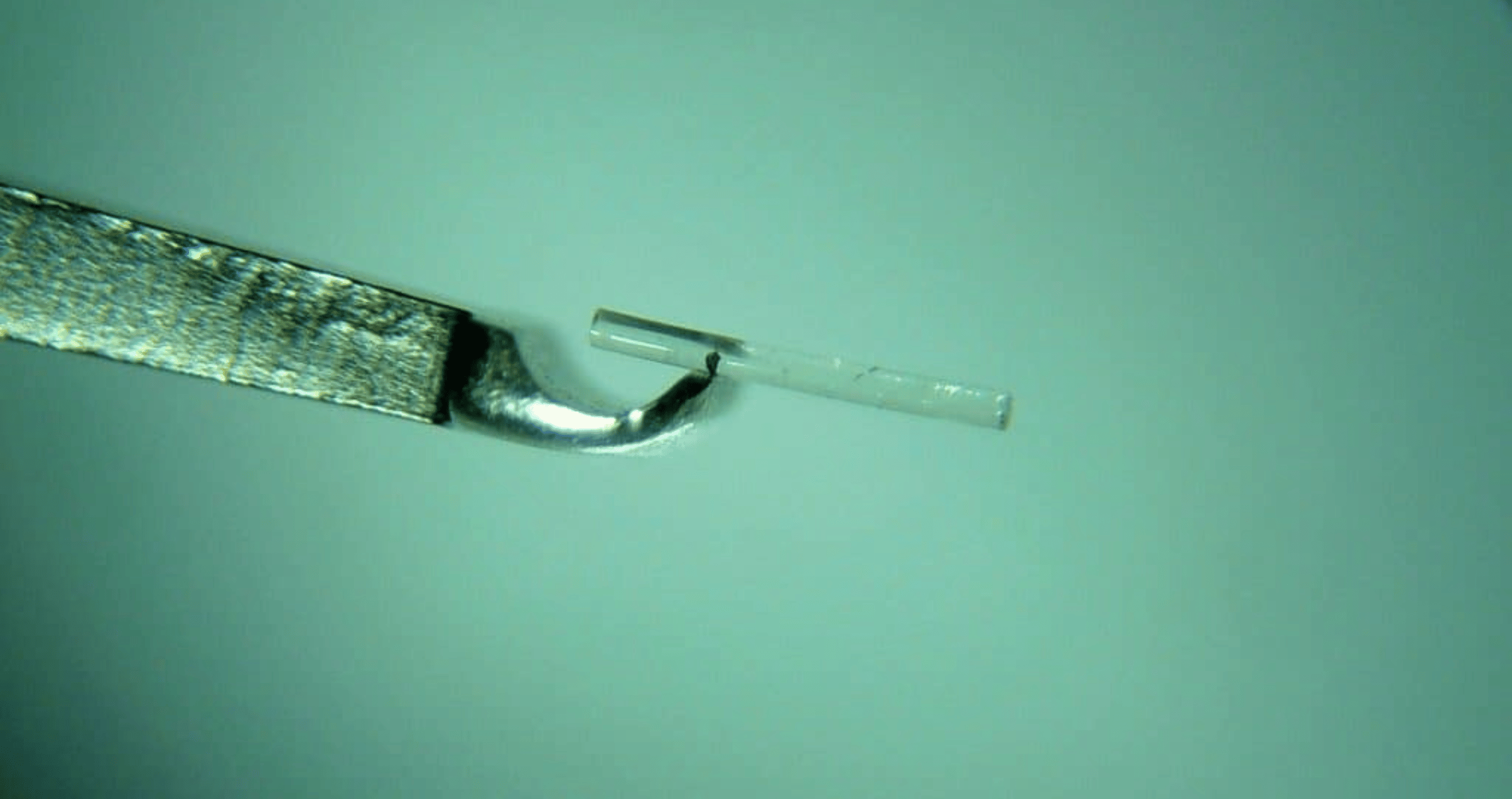
The interim results, from an ongoing phase 2a clinical study for the company’s PA5108 ocular implant in subjects with mild to moderate glaucoma, revealed a >20% reduction in intraocular pressure (IOP) at 12 weeks and 26 weeks in the initial cohort.
The ocular implant is designed to deliver six months of latanoprost and is biodegradable, which allows for repeat dosing.
In a further cohort, the implant’s rapid biodegradation profile has also allowed a repeat second dosing for eight out of 17 participants at 21 weeks, providing ongoing, uninterrupted therapy, with no product related adverse effects.
“For ocular implants to ultimately replace daily eye drops for the treatment of glaucoma, they need to be suitable for repeat administration, which has been a principal goal of PolyActiva’s development program. Our phase 2a results indicate we are well placed to meet that objective,” CEO of PolyActiva Ms Vanessa Waddell said.
Treating glaucoma with daily eye drops has been the mainstay of glaucoma therapy, however, between 41-90% of patients do not correctly administer their drops after one year, PolyActiva reported. Ineffective treatment and poor adherence can accelerate loss of sight.
“Sustained drug delivery via an implant could reduce the reliance on daily eye drops and ensure that the correct amount of medicine is delivered consistently, every day,” Waddell said.
“The implants received by these trial participants were present for 26 weeks before they biodegraded, which gives us confidence that our technology will be able to deliver a long-term treatment option for glaucoma patients. We look forward to continuing our development of this product as we plan for confirmatory trials in the US.”
PolyActiva presented its interim phase 2a data at the Eyecelerator Ophthalmic Innovation Conference in San Francisco on 2 November 2023. The company plans to initiate its phase 2b clinical trial in the US, Australia and New Zealand in 2024.