Share
Biotechnology company PolyActiva has completed patient enrolment for its Phase I clinical trial to study safety and tolerability of its biodegradable, slow-release ocular implant for the treatment of glaucoma.

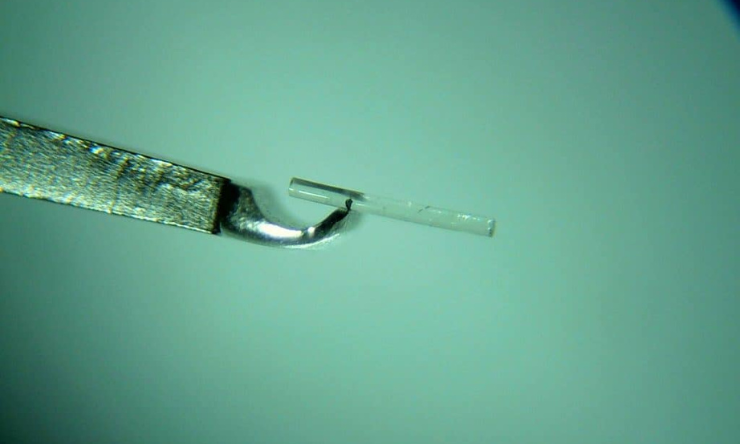
The implant has potential to revolutionise treatment for millions of glaucoma patients. Current glaucoma treatment requires eye drops to be administered multiple times daily. Instead the implant delivers medication to the eye for at least 30 weeks, and then biodegrades completely, leaving no residue. With compliance a prominent issue for glaucoma patients globally, this provides an effective alternative to having to administer drops.
"The PolyActiva implant is an exciting advance in glaucoma treatment. It is simple to administer, is well received by patientes and ensures the glaucoma medications is delivered to the eye continuously, each and
every day" says Dr Nathan Kerr, Principal Investigator for the Phase I study.
"To date, our Phase I study has demonstrated that our implantis safe and well tolerated and provides treatment for a six-month period before it biodegrades completely," says PolyActiva CEO Dr Russell Tait. "With recruitment now closed, top-line safety and initial efficacy data from this study will be available by the end of this calendar year"