Share
Government advisors have recommended minimally invasive glaucoma surgery (MIGS) should be publicly funded as a standalone procedure, following extensive campaigning from Australia’s ophthalmic community.

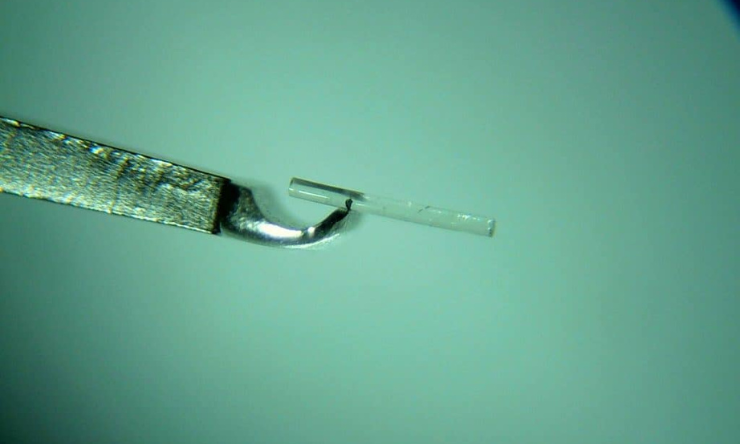
Almost a year since rejecting the proposal, the Medicare Services Advisory Committee (MSAC) now believes there is sufficient evidence for standalone trabecular micro-bypass glaucoma surgery (TB MBGS) – a form of MIGS – to have its own Medicare Benefits Schedule (MBS) item number.
It follows years of advocacy led by the Australian Society of Ophthalmologists (ASO) after a 2017 change in item number regulations threatened funding for all MIGS procedures.
The issue was partially addressed in October last year when the Federal Government approved a new item number, but limited the procedure’s availability to patients who were simultaneously undergoing cataract surgery.
The ASO was encouraged by the development and continued to campaign. It believed the move created two classes of glaucoma patients because those who did not need cataract surgery, or had already had the operation, would not be eligible.
According to MSAC, it originally did not support public funding of standalone MIGS due to unclear eligibility criteria and high ‘leakage risk’ to other populations, poor comparative safety data, poor comparative efficacy, and unsatisfactory economic assessment.
However, at a meeting in August, MSAC agreed to support MIGS in isolation after considering the strength of available evidence for comparative safety, clinical effectiveness and cost-effectiveness presented by the ASO. Its application was supported by the Australia and New Zealand Glaucoma Society, treatment companies such as Glaukos, health economists Thema Consulting and Glaucoma Australia.
ASO vice-president and Sydney glaucoma surgeon Dr Ashish Agar, from the Prince of Wales Hospital, told Insight the organisation was encouraged by the recommendation, which would now go before Federal Health Minister Mr Greg Hunt.
“It’s a very positive development. We thank the minister for his support as this would not have been forthcoming for a long time without his intervention, and we can now reassure our patients that there is a solution in sight” Agar said.
“This about giving the patients much better access, and it’s an important step on the way to restoring that equity.”
In its decision, MSAC compared MIGS alongside trabeculectomy and concluded that although MIGS was slightly less effective, it was safer and may allow some patients to delay or avoid trabeculectomy.
Other analysis shows that MIGS generally costs less than trabeculectomy, and is projected to save the MBS approximately $500,000 per annum by the fourth year of listing.
According to MSAC, this saving is driven by fewer initial and revision trabeculectomy services, however the true financial impact will depend on the efficacy of MIGS in reducing trabeculectomies, which is uncertain.
“MSAC will review the use of [MIGS] after two years to see what effect it has on the number of trabeculectomies being done and make sure it is still cost-effective,” the committee stated.
MSAC also supported establishing a registry to record outcomes, believing it would be important to monitor use of MIGS and rates of trabeculectomy over time.
A Federal Department of Health spokesperson told Insight that given the financial implications associated with new listings on the MBS, MSAC’s recommendations are subject to consideration by the government.
“This generally occurs through either the annual Budget or the Mid-Year Economic and Fiscal Outlook,” the spokesperson said.
“If the Government does agree to the listing, relevant stakeholders will be advised prior to listing to confirm the listing date and final item details.”