Share
Glaucoma is a neurodegenerative eye disease and one of the most common causes of blindness worldwide. It is characterised by the progressive loss of retinal ganglion cells and their nerve fibres which form the optic nerve.
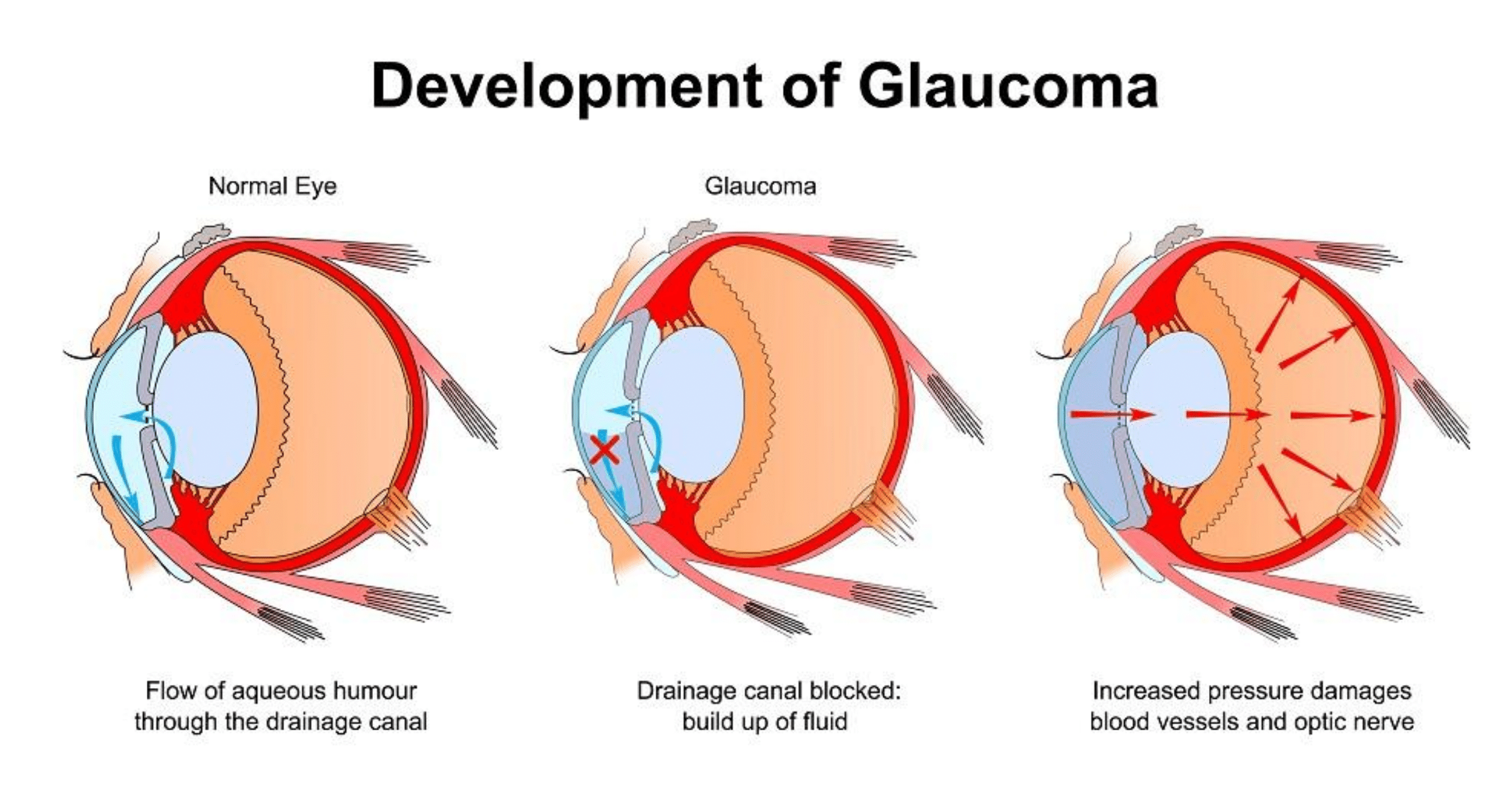
These changes lead to the progressive irreversible loss of peripheral vision with or without changes in intra-ocular pressure (IOP). Since IOP is the only treatable glaucoma risk factor, current medical and surgical treatments aims to decrease IOP to slow or prevent further vision loss. This can be achieved by decreasing production of fluid (aqueous) entering the eye and/or increasing its outflow. When patients do not respond well to maximally tolerated drug treatments, then laser and/or surgical treatments are considered. If however, traditional surgical methods are not effective in reducing IOP, patients have had very few options left, until now.
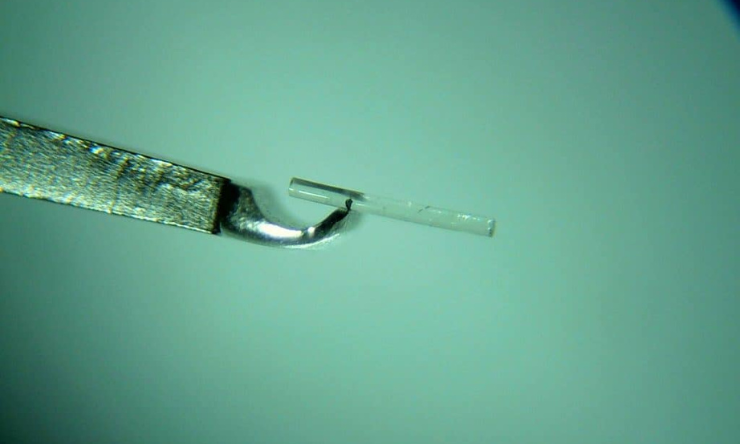
XEN is a flexible non-degradable gelatin shunt to be implanted in the sub-conjunctival space in the eye1-3. The 6mm shunt conforms to the ocular tissue, effectively creating a new drainage channel. Proper outflow of aqueous humour is enabled, therefore reducing IOP.
Countries currently in use of XEN
XEN was approved by the European Union, Canada, Switzerland, and Turkey, and approved by the U.S. Food and Drug Administration in November 2016.
Advantages
The shunt is soft and flexible when wet. The procedure is minimally invasive - with a single use, pre-loaded injector used to implant the device. Surgical and recovery times are shorter and less side effects than more invasive procedures such as trabeculectomy and tube shunt surgeries.
Suitable patients
Patients with uncontrolled glaucoma, primary open-angle glaucoma, and pseudoexfoliative or pigmentary glaucoma with open angles that are unresponsive to maximum tolerated medical therapy1,3.
Non-suitable patients
Patients with angle-closure glaucoma where the angle has not been surgically opened, previous glaucoma shunt/valve or conjunctival scarring/pathologies in the target quadrant, active iris neovascularisation, anterior chamber intraocular lens, intraocular silicone oil, and vitreous in the anterior chamber3.
Side effects and complications
May include: choroidal effusion, hyphema (blood in the anterior chamber of the eye), hypotony (very low IOP), implant migration, implant exposure, wound leak, the need for secondary surgical intervention, and intraocular surgery complications3.
Declaration
There is no conflict of interest.
Disclaimer
This article is for general information only. Please consult your ophthalmologist for medical advice.
News links:
http://eyewiretoday.com/2017/03/14/usc-roski-eye-institute-ophthalmologists-are-first-to-use-xen-gel-stent-to-treat-glaucoma-patients-in-los-angeles
http://hscnews.usc.edu/fda-approves-glaucoma-treatment-tested-at-usc-roski-eye-institute/
https://www.aao.org/headline/fda-approves-xen-gel-stent-glaucoma
http://www.glaucoma.org/news/allergan-receives-fda-approval-for-new-xen-glaucoma-treatment-system.php
http://www.prnewswire.com/news-releases/usc-roski-eye-institute-ophthalmologists-are-first-to-use-xen-gel-stent-to-treat-glaucoma-patients-in-los-angeles-300423412.html
References supplied