Share
MIGS has ushered in an entirely new field of interventional glaucoma treatments. We examine those currently available and look ahead to what is on the horizon in this rapidly advancing space.
As a glaucoma and cataract surgeon, I have greatly enjoyed the significant advances in technology and new surgical techniques with the introduction of minimally invasive glaucoma surgery (MIGS). These procedures promise better patient outcomes and an enhanced safety profile, allowing for intervention earlier in the disease process. The field is no longer in its infancy, with a strong and evergrowing evidence base supporting the use of these devices. In 2019, we have seen the publication of several landmark studies demonstrating unequivocal superiority of canal-based procedures over cataract surgery alone, as well as safety and efficacy data for subconjunctival procedures.
Approved MIGS
iStent And iStent Inject
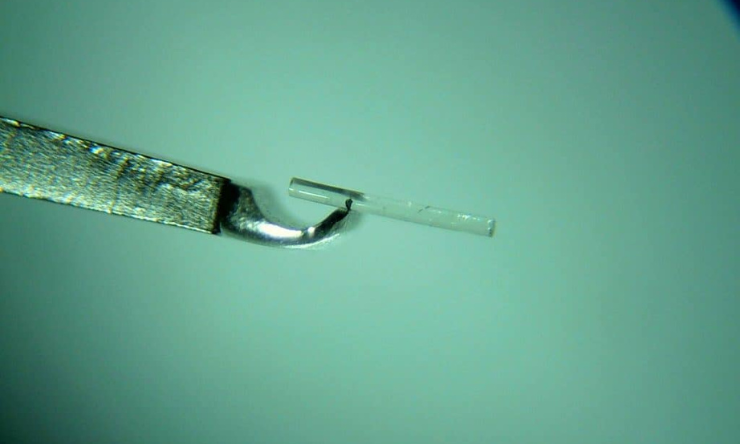
 Both of these devices optimise the natural physiological outflow of aqueous humour by creating a patent bypass through the trabecular meshwork into the Schlemm canal. The iStent (Glaukos) was the first device to be approved and heralded the introduction of MIGS. As good as the first generation iStent was, the second-generation iStent inject (Figure 1 and 2) represents an evolution in technology, promising better patient outcomes by including two stents for greater aqueous outflow as well as allowing for targeted placement, ideally over or near collector channels several clock hours apart. Due to enhanced speed and ease of insertion, most surgeons have transitioned or adopted the second generation system. Made of surgical grade titanium and measuring just 0.23mm x 0.36mm, the stents are implanted using an injector pre-loaded with two stents. Each stent is designed to carry the total amount of aqueous humour produced by the human eye (2.5μl/min).1 Typically performed at the time of cataract surgery, these devices provide a greater reduction in intraocular pressure (IOP) and/or need for glaucoma medication than cataract surgery alone.1,2 This can be beneficial for people with both cataracts and glaucoma, especially where IOP is sub-optimal or there is intolerance to, or side effects from, glaucoma eye drops. Importantly, trabecular stent implantation does not preclude additional medical or surgical therapy that may become necessary in the future.
Both of these devices optimise the natural physiological outflow of aqueous humour by creating a patent bypass through the trabecular meshwork into the Schlemm canal. The iStent (Glaukos) was the first device to be approved and heralded the introduction of MIGS. As good as the first generation iStent was, the second-generation iStent inject (Figure 1 and 2) represents an evolution in technology, promising better patient outcomes by including two stents for greater aqueous outflow as well as allowing for targeted placement, ideally over or near collector channels several clock hours apart. Due to enhanced speed and ease of insertion, most surgeons have transitioned or adopted the second generation system. Made of surgical grade titanium and measuring just 0.23mm x 0.36mm, the stents are implanted using an injector pre-loaded with two stents. Each stent is designed to carry the total amount of aqueous humour produced by the human eye (2.5μl/min).1 Typically performed at the time of cataract surgery, these devices provide a greater reduction in intraocular pressure (IOP) and/or need for glaucoma medication than cataract surgery alone.1,2 This can be beneficial for people with both cataracts and glaucoma, especially where IOP is sub-optimal or there is intolerance to, or side effects from, glaucoma eye drops. Importantly, trabecular stent implantation does not preclude additional medical or surgical therapy that may become necessary in the future.
The iStent inject system received CEmark certification in 2011, has been used in Europe since that time, and has been available more recently in Australia. Complementing over eight years of clinical experience and a considerable body of long term studies, the results of a pivotal randomised controlled trial supporting approval by the U.S. Food and Drug Administration (FDA) were published this year.1 People with mild to moderate primary open angle glaucoma (POAG) requiring cataract surgery were randomised to iStent inject implantation or no stent implantation at the time of cataract surgery. This study showed clinically and statistically greater reductions in IOP with iStent inject implantation with cataract surgery versus cataract surgery alone. In the iStent inject group, 75.8% of eyes experienced a 20% or greater reduction in IOP without medication at 24 months compared to 61.9% of eyes which did not receive a stent.1 Eyes which received an iStent inject were significantly more likely to be medication-free at two years (63.2% compared to 50%).1 The iStent inject provided excellent safety, and at 24 months, the overall rate of adverse events for the iStent inject group was similar to cataract surgery alone.1
Recognising the clinical benefit of transtrabecular stenting, the Medicare Benefits Schedule rebate was increased by Medicare in 2018 based on the recommendation of the Medical Services Advisory Committee. Eligible patients undergoing minimally invasive glaucoma surgery in conjunction with cataract surgery are now entitled to receive a rebate of AU$925.70 from Medicare.
Hydrus Microstent
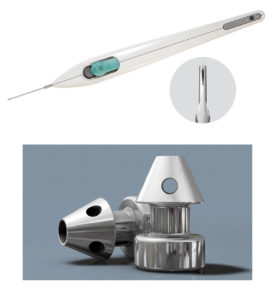
Another transtrabecular device, which aims to restore the eye’s natural aqueous outflow pathway, is the Hydrus Microstent (Ivantis, Figure 3). Commercially launched in Australia last year, the Hydrus Microstent is approximately the size of an eye lash and is made of a highly biocompatible and flexible material called nitinol. This flexibility enables the stent to be inserted through the trabecular meshwork into Schlemm canal, not only bypassing trabecular meshwork resistance but also scaffolding approximately three clock hours of Schlemm canal. The Hydrus Microstent therefore restores natural aqueous outflow in three ways – bypassing the resistance of the trabecular meshwork by creating a direct connection from the anterior chamber to Schlemm canal; scaffolding and dilating Schlemm canal open; and spanning 90 degrees to allow access to multiple collector channels. Because the stent is 8mm in length, it is easy to see whether it is in Schlemm canal, providing a high degree of surgical confidence. By covering an extended area, targeted placement over collector channels and multiple stents are not essential. The stent is not seen nor felt by the patient.
 The Hydrus Microstent has been shown to provide superior lowering of IOP and a greater reduction in glaucoma medication than cataract surgery alone.3,4 The HORIZON study was a landmark pivotal trial published last year and the largest MIGS trial to date. In this study, 556 people with mild to moderate open angle glaucoma were randomised to undergo cataract surgery with or without a Hydrus Microstent. This global study was conducted at 38 centres in nine countries in North America, Europe, and Asia. At two years, more than 77% of eyes treated with the Hydrus Microstent showed a significant 20% or more reduction in unmedicated IOP compared to 58% of the control group.4 On average, the Hydrus Microstent reduced IOP by 7.5 mmHg, approximately 2.3 mmHg (or 43%) more than cataract surgery alone.4 Follow up showed that 78% of Hydrus Microstent patients were medication-free at 24 months compared to 48% of controls.4 There were no serious ocular adverse events related to the microstent, and safety measures were similar between the two groups. Transient hyphaema occurred in 1.1% of Hydrus Microstent patients and peripheral anterior synechiae in 3.8%.4
The Hydrus Microstent has been shown to provide superior lowering of IOP and a greater reduction in glaucoma medication than cataract surgery alone.3,4 The HORIZON study was a landmark pivotal trial published last year and the largest MIGS trial to date. In this study, 556 people with mild to moderate open angle glaucoma were randomised to undergo cataract surgery with or without a Hydrus Microstent. This global study was conducted at 38 centres in nine countries in North America, Europe, and Asia. At two years, more than 77% of eyes treated with the Hydrus Microstent showed a significant 20% or more reduction in unmedicated IOP compared to 58% of the control group.4 On average, the Hydrus Microstent reduced IOP by 7.5 mmHg, approximately 2.3 mmHg (or 43%) more than cataract surgery alone.4 Follow up showed that 78% of Hydrus Microstent patients were medication-free at 24 months compared to 48% of controls.4 There were no serious ocular adverse events related to the microstent, and safety measures were similar between the two groups. Transient hyphaema occurred in 1.1% of Hydrus Microstent patients and peripheral anterior synechiae in 3.8%.4
The ground-breaking three-year results of the HORIZON study were presented in May of this year. At three years, 73% of Hydrus Microstent patients remained medication-free compared to 48% in the cataract only arm. But more significantly, there was a significant long term reduction in the need for major invasive glaucoma surgery in people treated with the Hydrus Microstent. This is the first time that early intervention with a MIGS device has been shown to reduce the need for invasive procedures such as trabeculectomy. Only 0.6% of Hydrus Microstent patients went on to have invasive surgery to control glaucoma, compared to 3.9% in the cataract only arm, an 85% reduction. Reassuringly, endothelial cell density remained stable and there was no significant difference between the Hydrus Microstent group and the cataract surgery only group at three years.
The Hydrus Microstent uses the same Medicare Benefit Schedule code as iStent when performed in conjunction with cataract surgery in eligible patients. Despite strong evidence that both iStent and Hydrus are effective when performed without cataract surgery,5,6 there is currently no item number for standalone use in Australia. As a result, patients are financially disadvantaged or must undergo more invasive, and higher risk procedures that they would otherwise not need to have. Patients, optometrists, ophthalmologists, and patient organisations are urged to speak with their member of parliament about this lack of access to care.
Ab-interno Canaloplasty (ABiC)
A new comprehensive minimally invasive canal-based glaucoma surgery is ab-interno canaloplasty (ABiC) (Ellex). Analogous to a cardiac angioplasty, this procedure uses a flexible microcatheter to re-establish the eye’s natural aqueous outflow system by viscodilating the trabecular meshwork, Schlemm canal, and the distal collector channels without damaging tissue and without leaving behind a stent or shunt. This procedure effectively reduces IOP and eliminates or reduces the glaucoma medication burden, and can be performed across the entire glaucoma disease spectrum – either in conjunction with cataract surgery or as a standalone procedure.
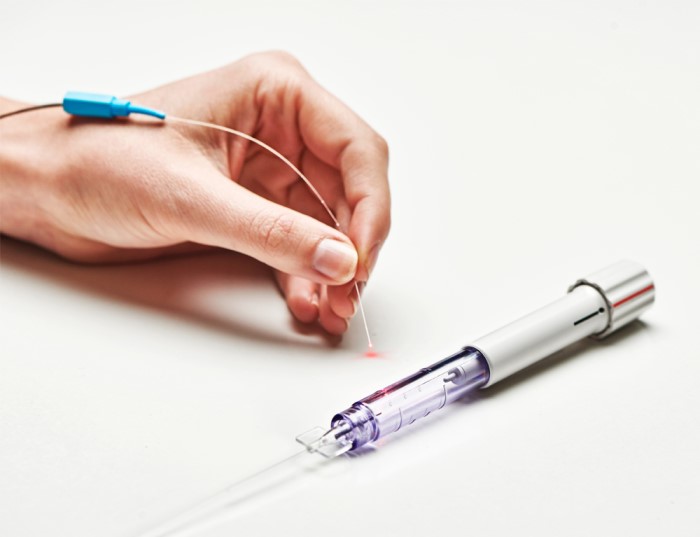
Ab-interno canaloplasty is performed through a clear corneal incision and an illuminated iTrack catheter is inserted through the trabecular meshwork into Schlemm canal and advanced 360 degrees around the entire canal. The tip of the canal has a rounded atraumatic tip to gently pass through the canal and an internal guidewire allows the catheter to pass through tight areas or push through herniations without the risk of creating a false passage. The tip of the iTrack catheter is illuminated to provide continuous location feedback, ensuring there is no inadvertent misdirection of the catheter into the suprachoroidal space or a collector channel. As the catheter is withdrawn, precise amounts of viscoelastic are injected using a ViscoInjector to dilate and clear the trabecular meshwork, Schlemm canal, and distal outflow pathways beginning with the collector channels. No tensioning suture or stent is required and the conjunctiva is preserved for future procedures should they be required in the future.
One of the major advantages of ABiC is that it addresses all points of obstruction in the normal outflow pathways and treats the entire 360 degrees of the canal, ensuring maximal effect. Additionally, it can be used in all stages of glaucoma – mild, moderate, or severe. ABiC can be performed with cataract surgery or as a standalone procedure in phakic or pseudophakic patients. As such, it is a very versatile procedure and can be performed in patients with glaucoma who are undergoing cataract surgery or as a dedicated standalone procedure where previous medical, laser, or surgical treatment has failed to adequately lower IOP. Like other canal-based procedures, safety is excellent and post-operative recovery is very rapid, making it suitable for use early in the disease process or in patients who are not ready for traditional surgery. There is no requirement for post-operative bleb management or manipulation, and as such this may be a good option for patients with conjunctival or ocular surface problems such as severe dry eye, pemphigoid, or conjunctival scarring from prior surgery.
There is emerging evidence from small clinical studies showing that ABiC provides significant and durable reductions in IOP as well as a reduction in medication dependence with or without cataract surgery.7–10 In a retrospective, comparative, consecutive case series of 75 eyes with uncontrolled POAG, ABiC in conjunction with cataract surgery (n=34) was associated with a reduction in mean IOP from 19.4 ± 3.7 mmHg on 2.6 ± 1.0 medications preoperatively to 13.0 ± 1.8 mmHg on 0.8 ± 0.2 medications at 12 months.8 In the stand-alone ABiC subgroup (n=41), the mean IOP decreased from 21.2 ± 5.3 mmHg on 3.0 ± 0.7 medications preoperatively to 13.7 ± 1.9 mmHg on 1.3 ± 1.1 medications at 12 months.8 There were no serious adverse events reported and post-operative recovery was rapid.8
Xen
[Editor's Update - The Allergen Xen Gel implant has been voluntarily recalled]
Transtrabecular procedures like the iStent inject or Hydrus Microstent provide excellent safety, in part due to downstream resistance providing protection against hypotony. This IOP ‘floor’ of around 15 mmHg makes them more suitable for patients with mild to moderate glaucoma or in patients where cataract surgery is required. Subconjunctival filtration surgery, by comparison, diverts aqueous humour from the anterior chamber to the subconjunctival space and can deliver very effective IOP lowering. This method of surgical drainage has been successfully used for over a century. New subconjunctival MIGS procedures have the potential to combine the safety profile of MIGS with the IOP-lowering ability of traditional glaucoma surgery.
 The Xen (Allergan) is a next-generation minimally invasive device that is inserted via an ab interno (or ab externo) approach to connect the anterior chamber and subconjunctival space, bypassing the conventional outflow pathway and creating a filtering bleb. The stent is 6mm long and is made of flexible and biocompatible porcine collagen. The Xen has an internal diameter of just 45μm to prevent hypotony and is designed to effectively, reliably, and predictably lower IOP while providing fast recovery, safety, and little tissue disruption. The Xen is indicated for glaucoma where medical therapy and/or prior surgical treatment (including other MIGS procedures) have failed to sufficiently lower IOP. The Xen is both approved and reimbursed in Australia for use with cataract surgery or as a standalone procedure in phakic or pseudophakic patients.
The Xen (Allergan) is a next-generation minimally invasive device that is inserted via an ab interno (or ab externo) approach to connect the anterior chamber and subconjunctival space, bypassing the conventional outflow pathway and creating a filtering bleb. The stent is 6mm long and is made of flexible and biocompatible porcine collagen. The Xen has an internal diameter of just 45μm to prevent hypotony and is designed to effectively, reliably, and predictably lower IOP while providing fast recovery, safety, and little tissue disruption. The Xen is indicated for glaucoma where medical therapy and/or prior surgical treatment (including other MIGS procedures) have failed to sufficiently lower IOP. The Xen is both approved and reimbursed in Australia for use with cataract surgery or as a standalone procedure in phakic or pseudophakic patients.
The results of the pivotal APEX trial were published this year and showed that the Xen effectively reduced IOP and medication burden in eyes with medically uncontrolled POAG.11 The results were similar if performed with cataract surgery or as a standalone procedure in phakic or pseudophakic patients.11 The safety profile in the study compared favourably to that published for trabeculectomy and tube shunts.11 At two years, 44.7% of eyes were medication-free.11 In another study, Xen was found to be effective as a standalone or combined procedure in both POAG and pseudoexfoliative glaucoma (PFXG).12 In this study, mean medicated IOP reduced from 19.8 } 5.8 mmHg and 19.7 ± 8.2 mmHg to 13.9 ± 4.6 mmHg and 13.6 ± 4.3 mmHg in the POAG and PXFG groups respectively at one year.12 Mean medications dropped from 1.9 ± 1.6 and 2.0 ± 1.3 preoperatively to 0.4 ± 0.8 and 0.5 ± 0.8 in the POAG and PXFG groups respectively.12 A previously published study showed there was no detectable difference in the risk of failure and safety profiles between standalone Xen with mitomycin C and trabeculectomy with mitomycin C.13 However compared to trabeculectomy, Xen provides faster visual recovery with less surgically induced astigmatism and requires fewer post-operative visits and in-clinic interventions.14 Surgical techniques for this procedure continue to evolve, to ensure predictable placement and results.
As a bleb-based procedure, pre-operative and post-operative management are important in obtaining optimum results. Ideally, topical glaucoma medications should be withheld or replaced with preservative-free glaucoma drops and topical steroids commenced at least one week prior to surgery to reduce conjunctival inflammation and hyperaemia. Mitomycin, although not licensed for use in glaucoma surgery, is routinely used to minimise conjunctival scarring and increase the rate of surgical success. However, this should be used carefully to reduce the risk of avascular blebs, which could predispose to infection.15 Lastly, as for trabeculectomy, needling may be required to enhance Xen outcomes by removing restrictions to aqueous outflow caused by fibrosis.
Pending MIGS Devices And Implants
PreserFlo Microshunt
The PreserFlo Microshunt (Santen) is a small tube that allows aqueous humour to drain from the anterior chamber to the sub-tenon space. Following a conjunctival dissection, the Microshunt is advanced ab externo into the anterior chamber through a scleral tunnel, thus eliminating the need for creation of a scleral flap, sclerostomy, iridectomy, scleral flap suturing, and postoperative suture lysis. The creation of a wide and deep sub-tenon pocket allows the posterior application of mitomycin soaked sponges. Made of an inert nonerodible polystyrene, the stent elicits very little foreign body reaction and does not require a scleral patch to prevent erosion. Not currently available in Australia, the Microshunt promises to provide trabeculectomy-like IOPs in a procedure which is considerably less invasive and requiring less post-operative intervention. I was fortunate to be an investigator in the pivotal trial for initial FDA approval at Moorfields Eye Hospital and gain surgical experience implanting the Microshunt. While the results of the FDA trial are still pending, smaller studies have shown reductions in IOP by 55% from baseline to 11.2 mmHg (personal communication, Paul Palmberg).
Suprachoroidal Device
The CyPass MicroStent (Alcon) was the first supraciliary stent to receive approval and enjoyed widespread adoption by glaucoma specialists worldwide. Approved on the basis of the COMPASS study, the device showed compelling efficacy with an average reduction in IOP of 7.4 mmHg and 85% of patients were medication-free at two years.16 However, in August 2018 Alcon voluntarily withdrew the device based on the COMPASS-XT study, which suggested that patients in the CyPass group had a higher rate of endothelial cell loss at five years, compared to patients who underwent cataract surgery alone. When it comes to patient safety, erring on the side of caution is preferred and Alcon should be commended for putting patient safety first. Baseline endothelial cell counts were 2,432 cells/mm2 for CyPass patients and 2,434 cells/mm2 in the cataract only group. There was no significant difference at two years, however at five years the endothelial cell count was 1,931 cells/mm2 in the CyPass group compared to 2,189 cells/mm2 in the control group. The rate of endothelial cell loss appeared to be related to stent position. When optimally positioned with no retention rings visible, the rate of endothelial cell loss was just 1.39% per year and not statistically different from cataract surgery alone. Even with one ring visible, the rate of cell loss was only 2.74% per year. However, when two or three rings were visible, the rate increased to 6.96% per year. It is important to note that none of the 505 participants developed permanent corneal oedema nor required a corneal transplant, even when three rings were present. To put these values in context, traditional trabeculectomy surgery with mitomycin is associated with endothelial cell loss of 10.0% at 12 months.17 Patients who have received the CyPass should be informed about the risk of endothelial cell loss in simple, non-technical language but reassured that the risk of any harm is low. Stent position should be documented and endothelial cell counts monitored by specular microscopy, where available, on an annual basis initially. If the stent is long and endothelial loss rates are concerning, the stent can be trimmed.
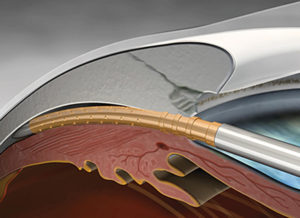 Since the withdrawal of the CyPass MicroStent, there has been a void in the supraciliary space. The iStent Supra (Glaukos) is a supraciliary device undergoing clinical trials. Made of polyethersulfone and titanium, the iStent Supra is shorter in length than the CyPass, which may be of benefit with regard to endothelial cell loss if it can be positioned further away from the cornea. Preliminary four year results in refractory glaucoma show promising results for the iStent Supra when used in combination with two iStents and medical therapy.18
Since the withdrawal of the CyPass MicroStent, there has been a void in the supraciliary space. The iStent Supra (Glaukos) is a supraciliary device undergoing clinical trials. Made of polyethersulfone and titanium, the iStent Supra is shorter in length than the CyPass, which may be of benefit with regard to endothelial cell loss if it can be positioned further away from the cornea. Preliminary four year results in refractory glaucoma show promising results for the iStent Supra when used in combination with two iStents and medical therapy.18
The MINIject (iStar Medical) is another supraciliary device undergoing investigation in clinical trials. Manufactured from a biocompatible silicone, the device is designed to promote integration with surrounding tissues while inhibiting fibrosis and preserving flow. It is inserted via an ab interno approach, similar to the CyPass, leaving only a small portion in the anterior chamber. Results are awaited with interest.
Sustained-release Drug Delivery
Adherence with glaucoma eye drops remains a significant challenge. Nonadherence with medical therapy 21 leads to faster progression and puts patients at greater risk of visual loss. In one study, 45% of patients used their drops less than 75% of the time, despite knowing they were being monitored.19 A new option coming on the horizon is sustained-release drug delivery, which may revolutionise the delivery of glaucoma medications. These devices, as well as selective laser trabeculoplasty (SLT), may work synergistically with MIGS devices or be an option for patients who do not require surgery but struggle with adherence or side effects from topical glaucoma medications.
The iDose (Glaukos) is a small titanium reservoir that is implanted in the angle. Filled with a formulation of Travoprost and capped with a membrane, the iDose is designed to allow continuous controlled drug elution into the anterior chamber over one year. By avoiding the need to penetrate the cornea, significantly lower doses of medication are required, resulting in fewer drug-related side effects. Once depleted, the implant can be removed and replaced. The iDose is currently undergoing clinical trials.
Bimatoprost SR (Allergan) is a biodegradable implant for the reduction of IOP that is injected into the anterior chamber using a 27-gauge needle. In phase I and II studies, bimatoprost SR provided IOP lowering similar to topical bimatoprost and at six months, 71% of eyes had not required re-treatment or rescue therapy.20 Adverse events were uncommon and typically transient.20 Conjunctival hyperaemia was infrequent but slightly more common in the implant group.20
The PolyActiva implant (PolyActiva) is another promising intracameral implant. This implant is designed to provide a constant daily therapeutic dose of latanoprost for at least 30 weeks, and then biodegrade, leaving no residue. I am the Principal Investigator for the phase I study which completed recruitment this year. The implant is simple to administer and has been well received by patients. The trial has demonstrated the implant is safe, well tolerated, and provides treatment for at least a six month period before rapidly biodegrading in less than six weeks.
Conclusions
The long-time paradigm of topical medications, followed by laser, and then conventional surgery is gone. The focus is now on safety, patient outcomes, quality of life, and cost-effectiveness. Recent studies support SLT as first line therapy 21 and now MIGS procedures are set to revolutionise the approach to the surgical management of glaucoma for many patients. No longer in its infancy, there is now strong evidence from large randomised controlled trials supporting the use of many MIGS devices, including that early intervention may delay the need for invasive surgery. It is important to note that not all MIGS devices are the same and no one device is universally applicable or effective in all cases. Similarly, a surgeon should base their treatment plan on what is best for the patient and not limit options to the procedures they perform. Traditional surgeries are highly effective and should be used where appropriate and without hesitation where clinically indicated. However, where traditional filtration surgery is not indicated or desired by the patient, consideration should be given to procedures which are less invasive, safer, and associated with faster patient recovery.
References:
1. Samuelson TW, Sarkisian SR, Lubeck DM, et al. Prospective, Randomized, Controlled Pivotal Trial of iStent inject Trabecular Micro-Bypass in Primary Open-Angle Glaucoma and Cataract: Two-Year Results. Ophthalmology 2019;126:811–821.
2. Samuelson TW, Katz JL, Wells JM, et al. Randomised Evaluation of the Trabecular Micro-Bypass Stent with Phacoemulsification in Patients with Glaucoma and Cataract. Ophthalmology 2011;118:459–467.
3. Pfeiffer N, Garcia-Feijoo J, Martinez-de-la-Casa JM, et al. A Randomised Trial of a Schlemm’s Canal Microstent with Phacoemulsification for Reducing Intraocular Pressure in Open-Angle Glaucoma. Ophthalmology 2015;122: 1283–1293.
4. Samuelson TW, Chang DF, Marquis R, et al. A Schlemm Canal Microstent for Intraocular Pressure Reduction in Primary Open-Angle Glaucoma and Cataract The HORIZON Study. Ophthalmology 2018;126:29–37.
5. Ahmed IK, Fea A, Au L, et al. A prospective randomised trial comparing Hydrus and iStent micro-invasive glaucoma glaucoma surgery implants for standalone treatment of open-angle glaucoma: The COMPARE Study. Ophthalmology 2019.
6. Kerr NM, Wang J, Barton K. Minimally invasive glaucoma surgery as primary stand-alone surgery for glaucoma. Clin Amp Exp Ophthalmol 2017;45:393–400.
7. Davids A-M, Pahlitzsch M, Boeker A, et al. Ab interno canaloplasty (ABiC)—12-month results of a new minimally invasive glaucoma surgery (MIGS). Graefe’s Archive Clin Exp Ophthalmol 2019:1–7.
8. Gallardo MJ, Supnet RA, Ahmed IK. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol 2018;12:2149–2155.
9. Gallardo MJ, Supnet RA, Ahmed IK. Circumferential viscodilation of Schlemm’s canal for open-angle glaucoma: ab-interno vs ab-externo canaloplasty with tensioning suture. Clin Ophthalmol 2018;12:2493–2498.
10. Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd 2018;32:223–227.
11. Reitsamer H, Sng C, Vera V, et al. Two-year results of a multicenter study of the ab interno gelatin implant in medically uncontrolled primary open-angle glaucoma. Graefe’s Archive Clin Exp Ophthalmol 2019;257:983–996.
12. Mansouri K, Gillmann K, Rao HL, et al. Prospective Evaluation of XEN Gel Implant in Eyes With Pseudoexfoliative Glaucoma. J Glaucoma 2018; 27:869–873.
13. Karimi A, Hopes M, Martin KR, Lindfield D. Efficacy and Safety of the Ab-interno Xen Gel Stent After Failed Trabeculectomy. J Glaucoma 2018;27:864–868.
14. Schlenker MB, Gulamhusein H, Conrad-Hengerer I, et al. Standalone Ab Interno Gelatin Stent versus Trabeculectomy: Post-Operative Interventions, Visual Outcomes, and Visits. Ophthalmol Glaucoma 2018;1:189–196.
15. Kerr NM, Wang J, Sandhu A, et al. Ab Interno Gel Implant-associated Bleb-related Infection. Am J Ophthalmol 2018;189:96–101.
16. Vold S, Ahmed IK, Craven RE, et al. Two-Year COMPASS Trial Results: Supraciliary Microstenting with Phacoemulsification in Patients with Open-Angle Glaucoma and Cataracts. Ophthalmology 2016;123:2103–2112.
17. Storr-Paulsen T, Norregaard J, Ahmed S, Storr- Paulsen A. Corneal Endothelial Cell Loss After Mitomycin C-augmented Trabeculectomy. J Glaucoma 2008; 17:654–657.
18. Myers JS, Masood I, Hornbeak DM, et al. Prospective Evaluation of Two iStent® Trabecular Stents, One iStent Supra® Suprachoroidal Stent, and Postoperative Prostaglandin in Refractory Glaucoma: 4-year Outcomes. Adv Ther 2018;35:395–407.
19. Okeke CO, Quigley HA, Jampel HD, et al. Adherence with Topical Glaucoma Medication Monitored Electronically The Travatan Dosing Aid Study. Ophthalmology 2009;116:191–199.
20. Lewis RA, Christie WC, Day DG, et al. Bimatoprost Sustained-Release Implants for Glaucoma Therapy: 6-Month Results From a Phase I/II Clinical Trial. Am J Ophthalmol 2017;175:137–147.
21. Gazzard G, Konstantakopoulou E, Garway-Heath David, Garg A, Vickerstaff, V,Hunter, R et al. Selective laser trabeculoplasty versus eye drops for first-line treatment of ocular hypertension and glaucoma (LiGHT): a multicentre randomised controlled trial. The Lancet. Volume 393, Issue 10180, P1505-1516, April 13, 2019