Share
From the lab to the clinic, scientists from the Centre for Eye Research Australia (CERA) are taking a multi-pronged approach to finding new treatments to save the sight of people with glaucoma.
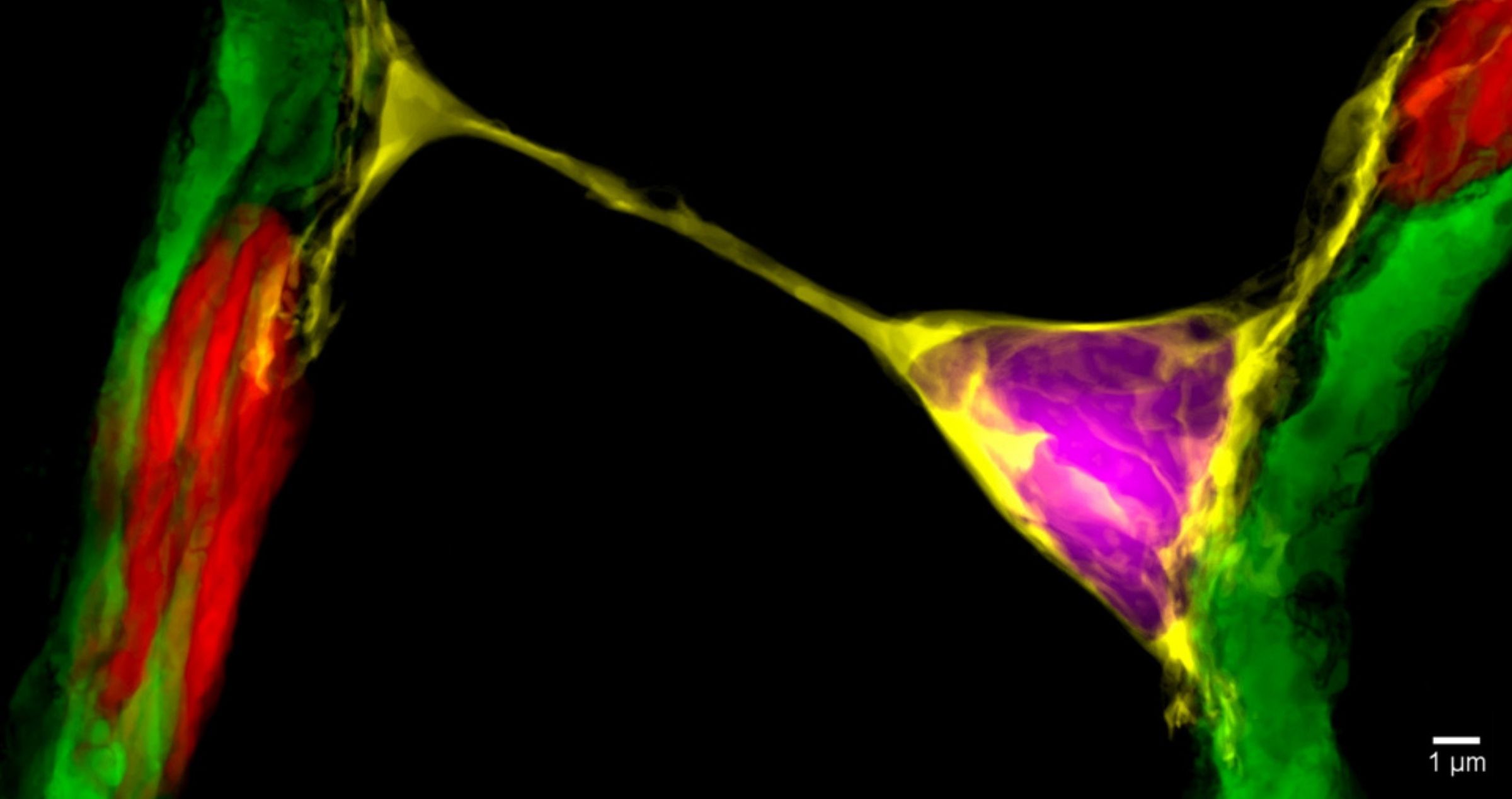
A nanotube image from Dr Alarcon-Martinez’s research.1
Every time we see a face, admire a beautiful view, or read a book, it’s the result of the successful interaction between millions of tiny cells and nerve fibres that send electrical signals between our eyes and brain.
When our vision works correctly, light is picked up by the retina at the back of the eye and turned into electrical signals which are transmitted by millions of retinal ganglion cells to the brain, helping us make sense of what we see.
Retinal ganglion cells transmit visual information to the brain via long nerve fibres, known as axons, which bundle together to make up the optic nerve.
The quality of images we see is influenced by how well these cells and their nerve fibres are working. In people with glaucoma, retinal ganglion cells and their nerve fibres that make up the optic nerve are damaged, leading to a decline in vision.
Glaucoma is often called the silent thief of sight because of the way it creeps up on patients. In the early stages of the disease, many people do not know they have a problem because the vision loss occurs in their peripheral vision.
Of the estimated 300,000 Australians living with the disease, about 150,000 don’t know they have it.
Preventing optic nerve damage
These efforts are important, because when glaucoma is detected early, the disease can be successfully managed and sight saved.
Glaucoma outcomes continue to improve thanks to research, but there are still about 15% of patients who don’t respond to current treatments.
That’s because although we know there are a range of risk factors for glaucoma – including age, genetics and elevated eye pressure – current treatments such as eye drops, laser and surgery focus only on lowering eye pressure.
We know that some patients with glaucoma continue to lose vision despite having normal eye pressure, but there are currently no treatments which aim to protect or repair damaged optic nerve cells. This means there is much more we need to do to find treatments that will save the sight of this group of patients.
Laying foundations in the lab
The first step to achieving this is having a better understanding of the complex processes – biochemical, molecular, cellular and genetic – that enable us to see, and why they stop working.
This knowledge is critical if we want to develop new treatments for glaucoma because it helps us understand what we are trying to fix. It could be a faulty gene that needs to be replaced, or a protein or molecule that can protect nerves and cells so they can function better.
At CERA, our basic scientists are examining key elements of the complex transport system that moves important molecules between the retina and the brain via the optic nerve, and we are investigating how we can recharge and reconnect cells to save sight.
In our Neurodegeneration Unit, Associate Professor Ian Trounce is investigating how changes in the DNA of the mitochondria, the tiny batteries which power our cells, could cause optic nerve damage. This research could lead to new approaches to slowing vision loss that go beyond reducing eye pressure.
My research group, a collaboration between the universities of Melbourne and Cambridge, is investigating potential gene therapies to strengthen the optic nerve and protect it from damage.
In one pre-clinical study, we used a gene responsible for producing a protein known as protrudin to produce a powerful result, which regenerated injured nerve cells and stopped them from dying.2
In another, we combined two key molecules to stimulate the growth of axons – the long nerve fibres which transport electrical signals along the optic nerve to the brain – to prevent vision loss and restore sight.3
Meanwhile, CERA’s newest research group, the Visual Neurovascular Research Unit, led by Dr Luis Alarcon-Martinez, is looking at another form of communication between cells that affects vision, examining the intricate processes that regulate blood supply to the cells of the retina.
Dr Alarcon-Martinez was part of a team from the University of Montreal which, for the first time, identified ‘inter-pericyte tunnelling nanotubes’ – tiny structures that help control the delivery of blood to retinal cells to keep vision intact.4
His continuing research at CERA will provide new insights into how we could potentially ‘recharge’ cells damaged by glaucoma to restore vision.
Early detection
As we increase our understanding of what causes glaucoma, there is also an opportunity to get better at identifying the early signs of disease. This will enable us to determine who is at most risk of vision loss so we can treat them before significant damage occurs.
CERA’s new Clinical Biomarkers team, led by Dr Zhichao Wu, aims to identify new clinical biomarkers for glaucoma. Dr Wu has already developed an international reputation as a macular researcher and I’m excited to see him expand the scope of his work to also investigate glaucoma.
This research is vitally important because the identification of new biomarkers can help fast-track the discovery of new sight saving treatments and bring them to patients sooner.
Currently one of the major barriers to finding new treatments is that clinical trials can take many years to assess a potentially promising new therapy. Identifying new biomarkers, which occur at an earlier stage of disease, can help solve this problem by enabling us to assess whether a new treatment is effective in a shorter timeframe.
Another barrier to early detection of glaucoma is access to screening services – and also the need for a more consistent, systemic approach across the eye care sector.
In 2022, we look forward to further progress in the development of Professor Mingguang He’s AI technology, which aims to increase access to eye screenings in remote and regional areas where there is a shortage of eye care professionals.
Meanwhile, our new Health Services Unit, led by Peter Larsen, will continue its research to improve early glaucoma detection rates and eliminate undetected glaucoma in our community.
Trialling new treatments
Clinical trials are the cornerstone of bringing new treatments to patients.
They provide the evidence we need to prove that a new device or drug is safe, effective and makes a difference for patients.
There has been much interest in the results of our promising trial of vitamin B3 to support the visual function of people with glaucoma.5
This year, I will join Dr Flora Hui to begin the next phase of the TAMING (Targeting Metabolic Insufficiency in Glaucoma) trial, which will involve patients in Adelaide and Melbourne.
By trialling the treatment on a larger group over a longer period, we hope to determine whether vitamin B3 should be taken on an on-going basis by patients, in addition to their regular treatments such as eye drops.
As there are currently no treatments to protect and strengthen the optic nerve, we hope vitamin B3 could help to not only protect against ongoing degeneration but also to improve the function of cells that have already been damaged.
From lab to clinic
Like much of our research, the TAMING trial is just one example of our ability to translate research that starts in the lab to a clinical trial.
The CERA team who began the first clinical study was inspired by pre-clinical research by colleagues in the United States,6 which showed vitamin B3 could protect the optic nerve in experimental models of glaucoma.
This demonstrates the value of CERA’s wide international collaborations, and also, of having in-house research teams spanning basic science to clinical research.
Together we can tackle complex diseases like glaucoma from many different angles – and move our research right through from bench to bedside.
This article has been republished courtesy of www.mivision.com.au
References
1. Alarcon-Martinez et al., 2020. Nature. 2020. 585(7823):91-95.
2. Petrova, V et al. Protrudin functions from the endoplasmic reticulum to support axon regeneration in the adult CNS. Nature Communications; 5 Nov 2020.
3. Khatib, TZ et al. Receptor-ligand supplementation via a self-cleaving 2A peptide-based gene therapy promotes CNS axon transport with functional recovery. Science Advances; 31 March 2021.
4. Alarcon-Martinez, L. et al Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature, 12 August 2020.
5. Hui F, et al. Improvement in Inner Retinal Function in Glaucoma with Nicotinamide (Vitamin B3) Supplementation: A Crossover Randomised Clinical Trial. Clinical and Experimental Ophthalmology. 28 July 2020.
6. Williams PA et al. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science; 17 Feb 2017.




