Share
A German medical device developer has implanted its first glaucoma patient with an eye pressure sensor, potentially revolutionising future self-management of the disease.
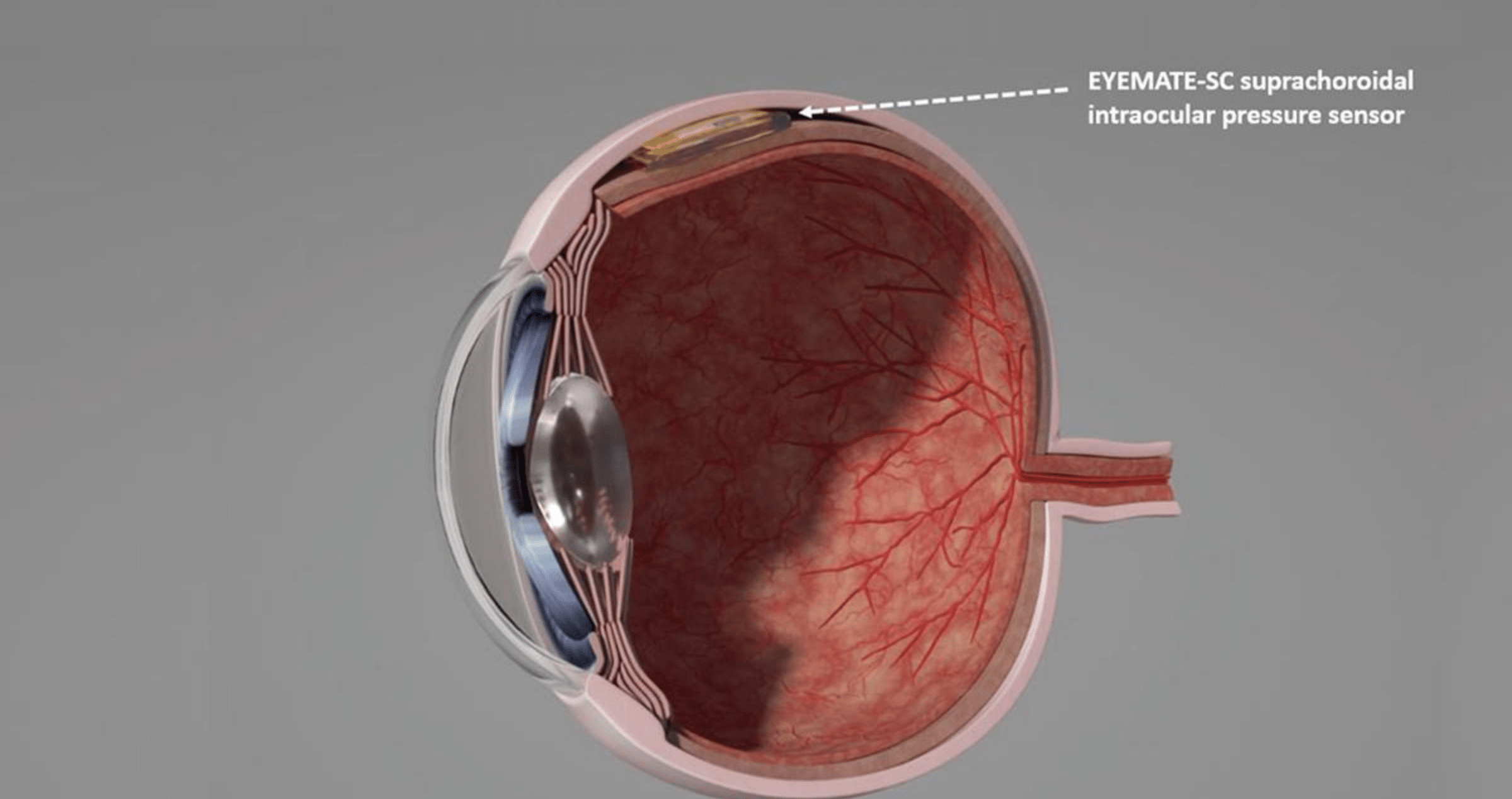
The procedure is part of Implantdata’s first-in-human clinical study to validate the EYEMATE-SC sensor implant, which resides in the eye and is designed to allow continual monitoring of intraocular pressure (IOP).
Principal investigator Professor Peter Szurman, of the Eye Clinic Sulzbach, Knappschaft Hospital Saar, performed the surgery, placing the device in the eye’s suprachoroidal in conjunction with non-invasive glaucoma surgery.
“The new Implandata sensor is pleasantly small and easy to surgically implant; therefore, most patients undergoing glaucoma surgery are likely to be eligible candidates for such a pressure sensor,” he said.
“This breakthrough product enables glaucoma patients for the first time to monitor their own eye pressure at any point in time. I expect that it will improve therapeutic compliance and also significantly reduce the risk of unnecessary visual field loss or even blindness due to glaucoma.”
The device is a follow up to the company’s CE-marked EYEMATE-IO intraocular sensor implant, which is used on glaucoma patients undergoing cataract surgery.
As current measurement methods can be sporadic and require in-office procedures conducted just a few times a year, implanted sensors that generate live, at-home IOP readings could be crucial to improving glaucoma self-management.
According to Implant data, earlier studies related to its EYEMATE-IO demonstrate at-home monitoring improves therapy compliance.
“EYEMATE’s remote patient care capabilities will result in more efficient disease management, as the number of office visits may be reduced for a considerable number of patients, while the eye doctor attains more and better information about the patient’s specific situation,” a company release stated.
“The aggregation of IOP measurement data may [also] shed new light on the emergence and progression patterns of the disease, potentially unlocking new or more efficacious intervention approaches.”
Implandata is expanding its study to include the Ophthalmic Clinic of Ruhr-University Bochum, the Department of Ophthalmology at University Mainz, and the Montchoisi Clinique Lausanne.
The Data Safety Monitoring Board, chaired by Professor Emeritus Günter Krieglstein – the former Director of Department of Ophthalmology of Medical University Cologne – will track the progress of the study, which should be completed by 2020.



