Share
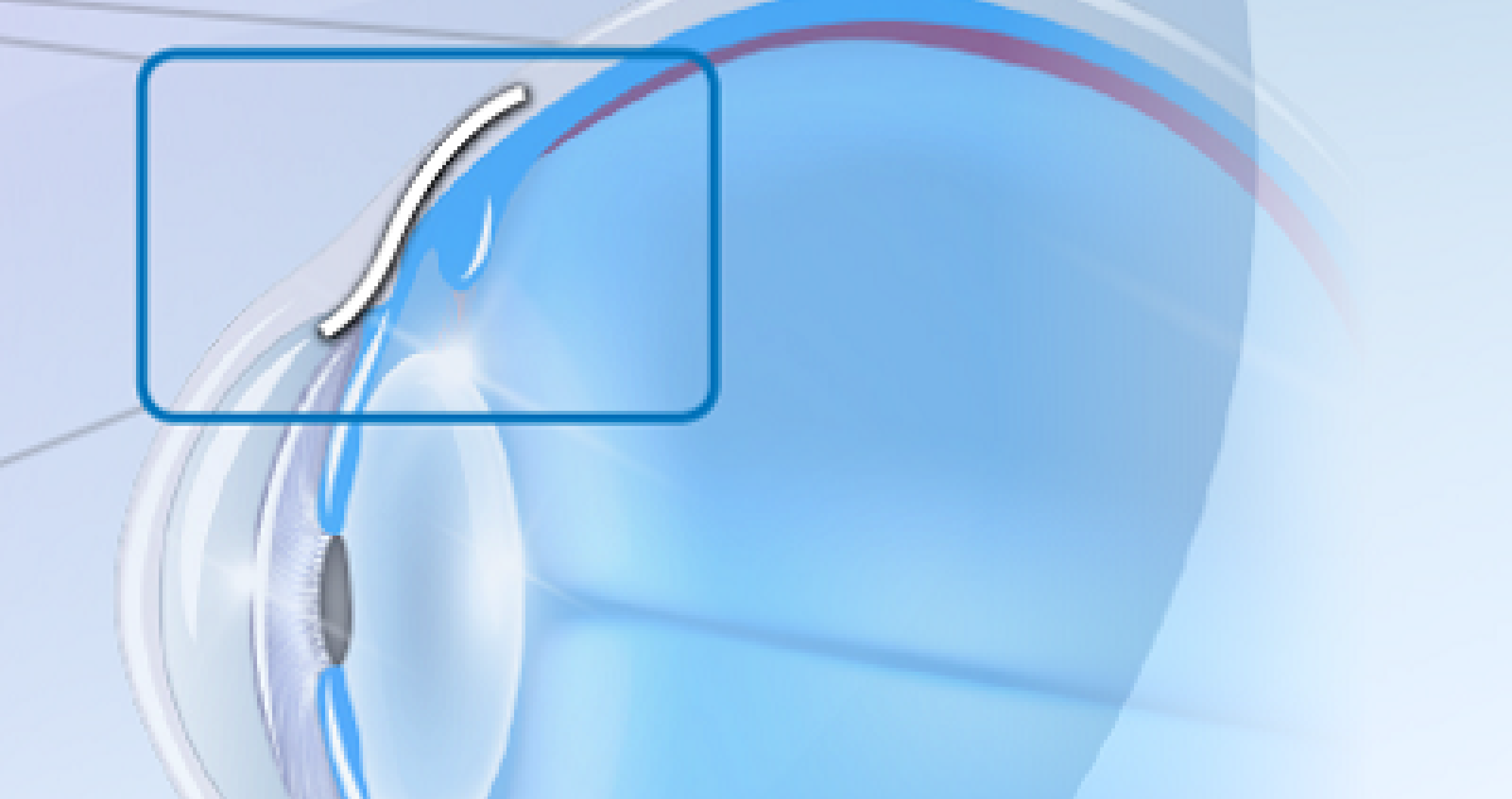
Allergan Australia Pty Ltd has made the decision to voluntarily recall all lots of XEN® Glaucoma Treatment System (XEN® 45 Gel Implant preloaded into a XEN® Injector) in Australia and New Zealand as part of a global recall.
The Therapeutic Goods Administration (TGA) in Australia and Medsafe in New Zealand the agencies responsible for the regulation and safety of therapeutic goods have been advised and Allergan are working closely with these agencies while conducting the recall.
Allergan’s top priorities are patient safety and product quality. The Company strictly follows Good Manufacturing Practice, which is a system for ensuring that products are consistently produced and controlled according to quality standards. During this process, some XEN® units were found not to meet the company’s strict standards of quality control.
Allergan has informed all the glaucoma surgeons across Australia and New Zealand who use XEN® of the global recall and asked them to postpone any planned surgery with the product whilst the Company works to resolve the situation.
For anyone who has already undergone XEN® implant surgery, post-operative follow-up regime will not change, and monitoring will continue as per the local standard of care. Patients that have received a XEN® Gel Implant are advised to speak with their healthcare professional should they have any questions.
Adverse events should be reported to the local Allergan office, Australia 1800 252 224 or New Zealand 0800 659 912, or email